

Was proposed, and a rate-determining step was also discussed. A mechanism for the electrocatalytic oxidation of methanol/ethanol Rotating disk electrode results obtained with a Ni(II)(DMG)2 coated graphite disk electrode showed that the electrocatalytic oxidation of alcohol is a 4-electron process producing formateĪnion (methanol oxidation) or acetate anion (ethanol oxidation).
The electrocatalytic oxidation currentsĬonsistently increase with the increase in Ni(II)(DMG)2 loading, OH−, and alcohol concentrations. − showed a strong catalytic activity toward electro-oxidation of methanol and ethanol. The electrochemical reaction was originally assumed to be a one-electron process converting It is necessary to cycle the electrode potential to a high value (e.g. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution.Nickel-dimethylglyoxime complex (abbreviated as Ni(II)(DMG)2) modified carbon paste and graphite electrodes were prepared by mixing Ni(II)(DMG)2 with graphite paste, and coating Ni(II)(DMG)2 to the graphite surface. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The anhydrous salt is yellow, but the more familiar hydrate NiCl 2 6H 2 O is green. Nickel(II) chloride (or just nickel chloride), is the chemical compound NiCl 2. For example, an aqueous solution of Ni(H 2 O) 62+ has a light green color, but Ni(NH 3) 62+ is deep blue. Because the distance in orbital splitting varies with ligand strength, a coordination complex with the same metal center can have a variety of colors based upon the coordinating ligand. The reason for this behavior is the presence of one or more unpaired electrons in the outer orbital shell. A paramagnetic element becomes magnetic in the presence of a magnetic field, but it loses its magnetic properties as soon as you remove the field. The list of paramagnetic atoms is much longer. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts.

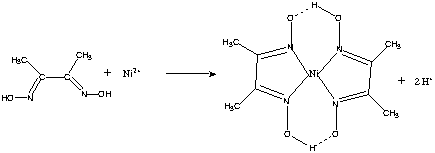
DmgH 2 is used in the analysis of palladium or nickel. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl).

Its abbreviation is dmgH 2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. Most interesting thing now would be to know PtCl42– and PdCl42–, Pd(NH3)42+, Pt(NH3)4. Whereas Mn(CN)63– has “d2sp3″ hybridisation and # 2 unpaired electrons Ni(DMG)2 is neutral chocolate- red complex having dsp2 and all paired electrons.


 0 kommentar(er)
0 kommentar(er)
